Abstract
Background: Hairy cell leukemia (HCL) is an uncommon B-cell malignancy characterized by pancytopenia on presentation. While the purine nucleoside analogues (PNAs) cladribine and pentostatin have resulted in long overall survival, infections remain a major cause of mortality. PNAs are known to be immunosuppressive, and in combination with the inherent infection risk of HCL, patients are vulnerable to serious and/or opportunistic infections throughout the disease course. Furthermore, there is prolonged CD4+ lymphopenia post-PNA treatment for 1-3 years. We thus conducted a comprehensive retrospective review at an academic center to (1) describe the incidence and types of infections seen in HCL and (2) determine how treatment with pentostatin versus cladribine may affect infection risk.
Methods: We reviewed charts of all HCL patients that were seen at The Ohio State University between January 1 2012 and June 30 2020. Patients with less than 1 year of follow up were excluded. We defined our infections of interest as (1) serious i.e. requiring hospitalization or (2) opportunistic i.e. organisms commonly seen in immunosuppression. These patients’ baseline demographics, disease characteristics, bone marrow biopsies, and treatment histories were abstracted from medical records. Time to infection was calculated from the start of HCL treatment to the onset of infections, treating death without infection as a competing risk. The cumulative incidence of infection was estimated, and the associations with patient characteristics were evaluated using Fine and Gray sub-distribution hazard models accounting for competing risks. Overall survival was calculated from diagnosis to date of death or last contact. Kaplan-Meier method was used to estimate the survival functions and corresponding 95% confidence intervals.
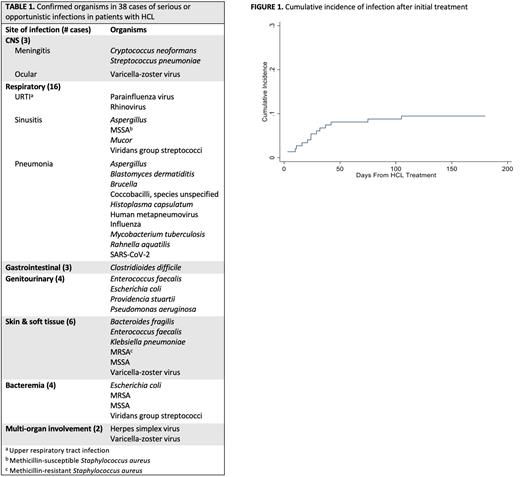
Results: Our study included 149 patients with a median follow up time of 6.9 years (range 1.3 - 48.8) and a 10-year estimated overall survival of 99% (95% CI: 93 - 100%). Median age at diagnosis was 51 years (range 22 - 80) and most patients (n = 117, 78.5%) were male. A total of 138 (92.6%) had classical HCL and 11 (7.4%) had variant HCL. There was a total of 70 incidents of infection, with 52 patients (35%) experiencing at least one infection and 18 (12%) with a second infection. Forty-six infections (66%) were serious and 24 (34%) were opportunistic. Most infections were likely to be seen 2 months prior to (n = 10, 14%) or 2 months after (n=14, 20%) initial treatment. The cumulative incidence of infection increased during the 50 days after starting treatment (Figure 1). Based on infection site, clinical presentation, and/or laboratory findings, 36 infections (51%) were classified as bacterial, 14 (20%) were viral, 8 (11%) were fungal, and 1 was concurrent viral and bacterial. Eleven (16%) infections did not have a confirmed organism. Sixty-seven (96%) infection cases had resolved; the outcomes in 2 cases were unknown, and 1 case resulted in death. Organisms were confirmed in 38 cases and are listed in Table 1. We examined baseline factors that could be associated with increased risk of infection, including sex, age at diagnosis, variant histology, total number of HCL treatment courses, prior infection, and a palpable spleen at diagnosis: none of these were associated with increased risk. Using cladribine as a reference, no treatment agents (including newer, non-PNA therapies) were associated with lower or higher infection risk. Examining the PNAs specifically, treatment with pentostatin versus cladribine was not associated with a significant difference in infection incidence (HR 1.12, 95% CI: 0.48 - 2.63).
Conclusions: In our large institutional cohort of patients with HCL, a considerable fraction (35%) of patients experienced serious or opportunistic infections. The majority of these were in the 2 months before or after initial treatment. We did not identify any demographic or disease factors associated with infection, although this is limited by the small sample size. Notably, treatment with either cladribine or pentostatin was not associated with a significant difference in infection incidence.
Disclosures
Kapoor:Hairy Cell Leukemia Foundation: Other: Principal Investigator. Blachly:KITE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; MingSight Pharmaceuticals: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; INNATE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board. Grever:AstraZeneca: Consultancy; Ascerta: Consultancy; Axio: Consultancy; Serono: Consultancy; Hairy Cell Leukemia Foundation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Consultancy; Pharmacyclics: Consultancy. Rogers:Pharmacyclics: Consultancy; Innate Pharma: Consultancy; Janssen: Research Funding; Novartis: Research Funding; AstraZeneca: Consultancy, Other: Travel Funding; Beigene: Consultancy; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal